Real-time Process Management Solutions

Our Real-time Process Management (RPM™) Systems establish a new benchmark for real-time process control in downstream bioprocessing by integrating of the CTech™ FlowVPX® in-line UV-Vis spectrophotometer into the KrosFlo® TFF Systems. This innovative solution empowers users to achieve unparalleled control and optimization of UF/DF processes, driving efficiency, quality, and innovation in biomanufacturing.
Achieving PAT-Driven Control with Real-Time Concentration Analytics
Introducing our groundbreaking Real-time Process Management (RPM™) line of TFF instruments, a cutting-edge solution designed to revolutionize process control in tangential flow filtration (TFF) systems. At the core of this innovation lies the integration of the CTech™ FlowVPX® in-line UV-Vis spectrophotometer into our KrosFlo® TFF Systems, providing users with unprecedented real-time insights and control over ultrafiltration/diafiltration (UF/DF) processes during downstream bioprocess development.
Real-time Concentration Measurement: Seamlessly integrated into our KrosFlo TFF Systems, the FlowVPX in-line spectrophotometer offers immediate and accurate concentration measurements directly within the process stream. This real-time data acquisition capability enables continuous monitoring of concentration changes throughout the UF/DF process.
PAT-Driven Control: Leveraging Process Analytical Technology (PAT) principles, our RPM™ Series drives process control based on real-time concentration data obtained from the FlowVPX device. Through closed-loop control systems, our TFF systems autonomously adjust process parameters in response to direct, real-time concentration measurements, ensuring precise control and optimization.
Optimized Efficiency and Quality: With the integration of the FlowVPX spectrophotometer and PAT-driven control, our KrosFlo TFF Systems ensure optimal efficiency and product quality. Operators achieve precise concentration targets consistently, reducing processing time and minimizing product variability, leading to higher throughput and superior product quality.
Reduced Risk and Enhanced Reliability: The RPM™ Series mitigates operational risks associated with manual sampling and off-line analysis by providing continuous, real-time monitoring of concentration levels. This proactive approach enables early detection of deviations from target concentrations, minimizing the risk of product deviations or failures. Additionally, the integration of the CTech FlowVPX in-line spectrophotometer into our KrosFlo TFF Systems ensures reliable concentration measurements, enhancing process consistency and product quality assurance.
Seamless Technology Integration: Seamlessly integrating the CTech FlowVPX in-line spectrophotometer into our KrosFlo TFF Systems, the RPM™ Series provides users with a comprehensive solution for real-time process management. This integration simplifies workflow and enhances usability, facilitating smooth scale-up and technology transfer across different production environments.
Empowering Innovation: Our RPM™ Series signifies a significant advancement in bioprocess monitoring and control, empowering industries to innovate and excel in bioprocessing applications. By harnessing the capabilities of in-line spectrophotometry and PAT-driven control, users unlock new levels of efficiency, productivity, and product quality in their UF/DF processes.
The Window into the Process
CTech™ FlowVPX® System: The Window into Your Process
Our Real-time Process Management (RPM™) Systems revolutionize process analytical technology in bioprocessing, made possible by the exclusive integration of the CTech™ FlowVPX® in-line spectrophotometer. These innovative systems provide continuous visibility and real-time concentration measurement during the ultrafiltration/diafiltration (UF/DF) process, empowering users with unmatched control and efficiency. Utilizing Variable Pathlength Spectroscopy, the FlowVPX® System ensures enhanced product consistency and quality, maximizes process efficiency, and eliminates the need for time-consuming dilution and background correction steps, saving valuable time and resources. Through in-line measurements offering immediate feedback, the UF/DF process can proceed seamlessly without interruptions for off-line analysis, thereby eliminating potential errors. As a result, your UF/DF process runs smoothly, ensuring superior efficiency and quality outcomes.

PAT-Driven Control with Integrated RPM Systems
Explore our innovative Real-time Process Management (RPM™) Systems, each purposely engineered to revolutionize process analytical technology for bioprocessing with the exclusive integration of the CTech™ FlowVPX® in-line spectrophotometer into our KrosFlo® TFF Systems. These systems empower users with unparalleled real-time insights and PAT-driven control over ultrafiltration/diafiltration (UF/DF) processes, accommodating diverse scale requirements and process demands.
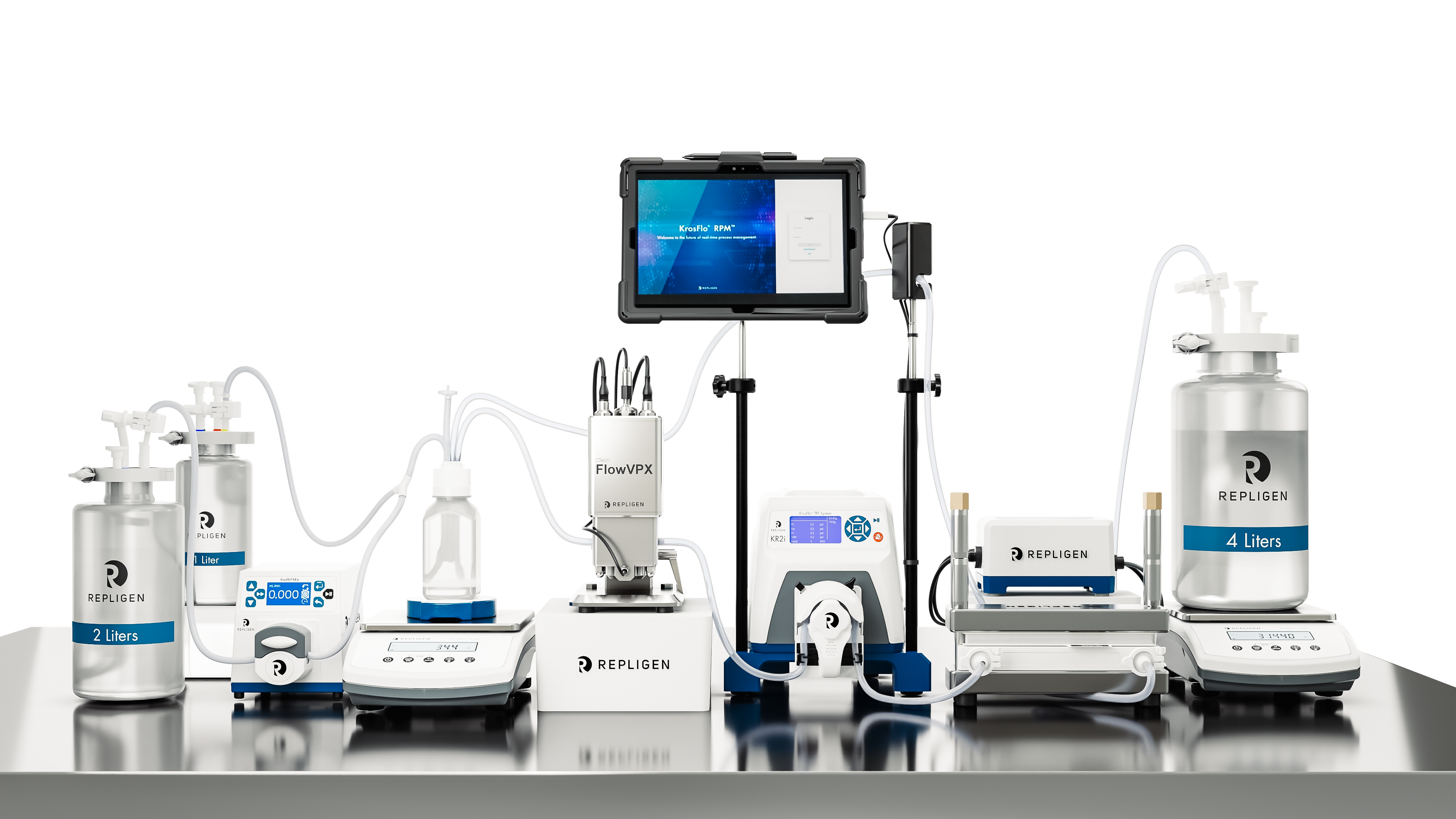
KrosFlo® KR2i RPM™ System
Ideal for lab-scale process development ranging from 2mL to 15L, the KR2i RPM™ System is a single-use, fully integrated benchtop TFF system controlled by real-time, in-line concentration measurement. Supporting both hollow fiber and flat sheet filters, it offers versatility in process design. Driven by a peristaltic pump, this system ensures precise and consistent fluid handling, enabling complex applications to run in fully-automated modes.
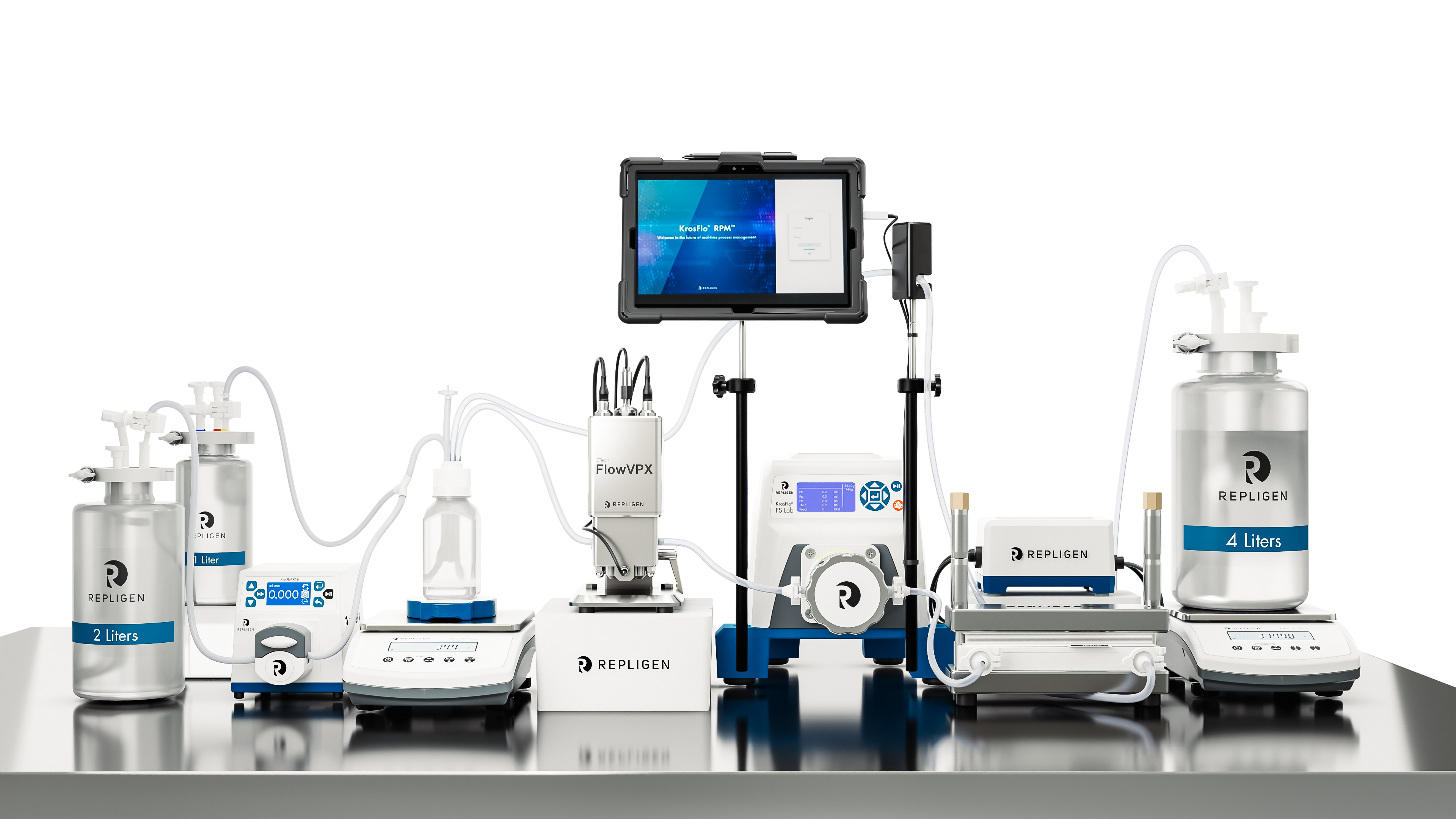
KrosFlo® FS 15 RPM™ System
Designed for lab-scale process development ranging from 150 mL to 15 L, the FS 15 RPM™ System is another single-use, fully integrated benchtop TFF system with real-time, direct concentration measurement. Utilizing a Diaphragm Pump, it offers efficient fluid transfer and control, contributing to enhanced process performance. Supporting both hollow fiber and flat sheet filters, it provides flexibility in membrane selection for diverse applications.
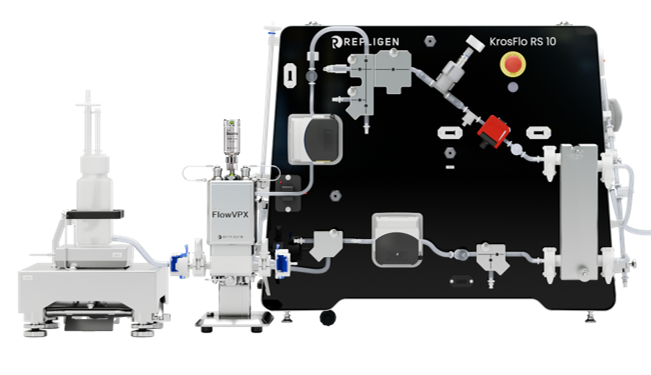
KrosFlo® RS 10 RPM™ System
For bench-scale cGMP production with end-to-end automation, The RS 10 RPM system adapts to your process requirements, offering the flexibility needed for efficient production. This system offers customizable pump options, including Maglev centrifugal pump, diaphragm pump, or peristaltic pump. Driven by in-line UV-vis concentration measurement, it ensures precise control over process parameters, optimizing productivity and product quality.
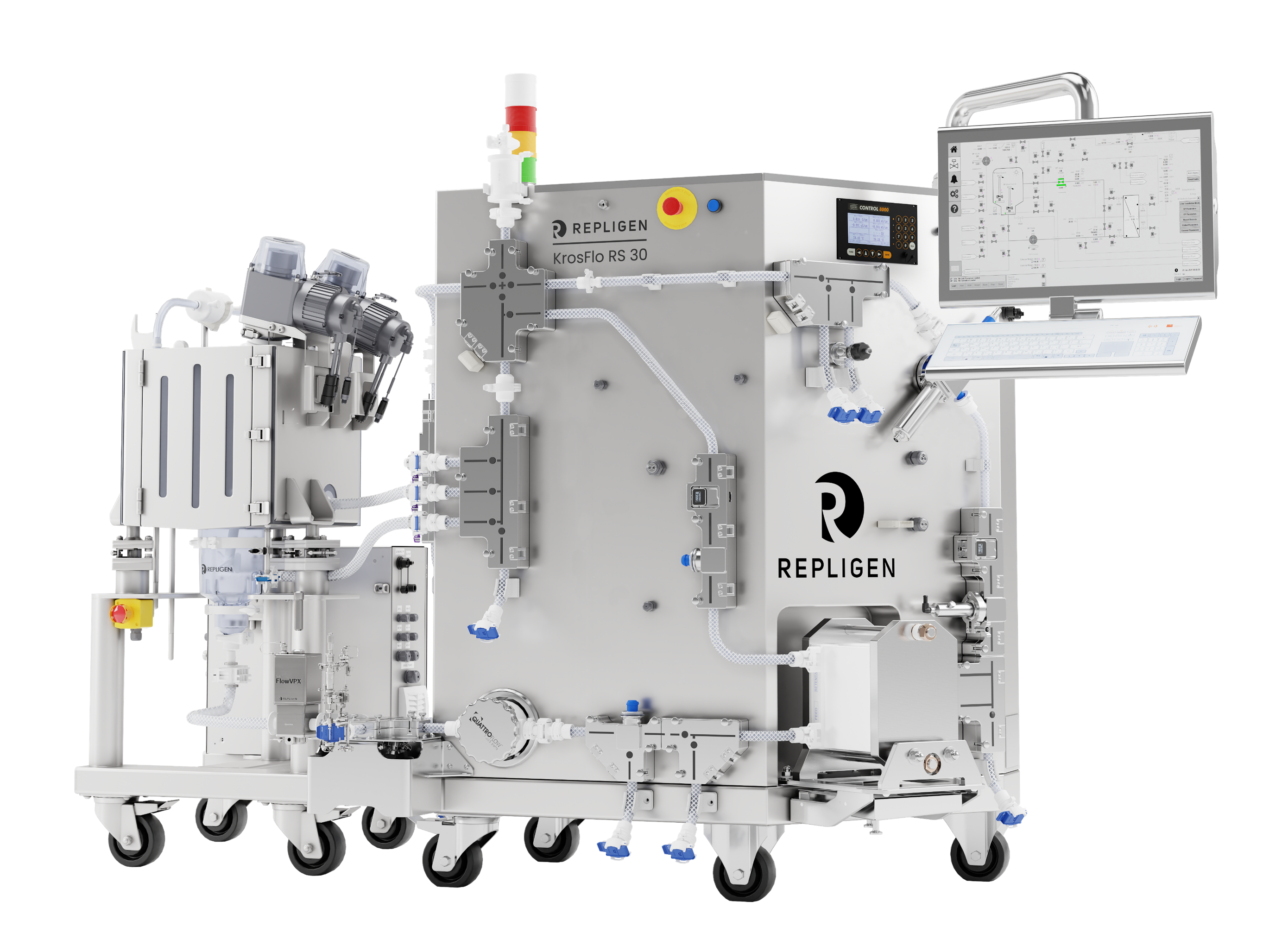
KrosFlo® RS 30 RPM™ System
A closed-loop solution where automation controls the entire bioprocessing journey based on real-time concentration data. The innovative RS 30 RPM system confidently expands production capabilities, scaling from 1.5L to 150L. By automating the process from sanitation to recovery, it eliminates batch-to-batch variation and manual intervention. Driven by continuous concentration measurement, it ensures that target concentrations are achieved consistently without interruption for off-line measurements, promising reliable and reproducible results every time.
Flexible System Components for Optimal Performance
All of our RPM™ Systems support either flat sheet cassettes or hollow fiber filters, providing versatility in membrane selection to meet specific process needs. With the support of Repligen's extensive membrane offerings, users can achieve precise control, efficiency, and reliability in their bioprocessing operations.
Enhance your TFF solution with simple connectivity using ProConnex® Flow Paths. These single-use flow paths are designed to streamline your processes ensuring reliability, reproducibility, and time savings.
TangenX® 平板膜包
This portfolio of single-use and reusable TFF flat sheet cassettes offer reliable, scalable, and cost-effective solutions for efficient ultrafiltration and diafiltration processes. From the TangenX SC TFF device’s holderless design that eliminates the need for holders and torquing; to the SIUS Gamma single-use, closed, sterile devices, to the PRO reusable cassettes, Repligen offers a TFF solution to meet the specifications of your process.
Spectrum® 中空纤维过滤器
Engineered for tangential flow filtration, Spectrum Hollow Fiber filters are packed in housings designed to handle high pressures. With a choice of fiber chemistries and sizes, from process development to manufacturing, these filters ensure optimal performance across various applications.
ProConnex®
流路
Our single-use flow paths are engineered for optimal performance, reproducibility, and time efficiency in TFF solutions. Constructed with Class VI Bioprocess-grade materials, they prioritize process requirements and sterility, ensuring swift and dependable bioprocessing operations. Supported by a supply chain with best-in-class lead times and a 95% on-time delivery record, our designs are fortified with robustness, redundancy, and risk mitigation measures.